Background: Patients with hematologic malignancy (HM) are hypothesized to be at high risk of poor outcomes with coronavirus disease 2019 (COVID-19), due to disease and therapy-related immunosuppression. Despite this, there are minimal reported data describing the outcomes of HM patients with COVID-19.
Methods: The COVID-19 and Cancer Consortium (CCC19) (NCT04354701) is an international registry aimed at investigating the clinical course and complications of COVID-19 in patients with cancer. The CCC19 cohort includes patients with active cancer or a history of cancer with presumed or laboratory-confirmed COVID-19. The registry contains de-identified patient demographics, information regarding cancer diagnosis and treatment history, COVID-19 treatments, and clinical outcomes. Patients greater than 18 years of age with laboratory-confirmed, symptomatic COVID-19 and HM diagnoses were included in this study. The primary outcome is a composite of severe COVID-19 illness (composed of mechanical ventilation, severe illness requiring hospitalization, intensive care unit (ICU) requirement, or death); mechanical ventilation, ICU level of care, supplemental oxygen, and 30-day mortality are reported as secondary outcomes. Baseline characteristics are reported for the entire cohort. Reported clinical outcomes are stratified by cancer type, cancer status, line of therapy received (never treated, first, second or later), receipt of cellular therapy or transplant (none, within 12 months, >12 months prior to COVID-19 diagnosis), last receipt of cytotoxic therapy (within 4 weeks, 1-3 months, 3-12 months prior to COVID-19 diagnosis), and receipt of HM therapies under investigation as repurposed treatments for COVID-19 (Bruton tyrosine kinase (BTK) inhibitors, Janus kinase (JAK) inhibitors, Bcr-Abl kinase inhibitors).
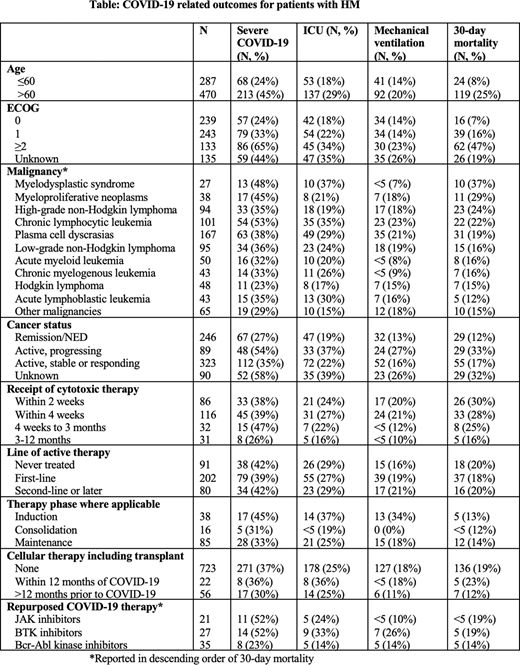
Results: From March 17, 2020 to July 31, 2020, a total of 757 patients with HM and COVID-19 were enrolled and met inclusion criteria. Median follow-up was 30 days (IQR 17-70 days). Median age was 65 years (IQR 55-75), 62% (470) were over age 60, 57% were men, 45% were non-Hispanic white (22% Black, 18% Hispanic, 5% other), 39% were former or current smokers, 27% were obese, 18% had Eastern Cooperative Oncology Group (ECOG) performance status ≥2, and 51% were on active treatment within 3 months of COVID-19 diagnosis.
Among patients with HM, 281 (37%) developed the primary endpoint. Five-hundred and eleven patients (67%) were hospitalized (some with non-severe disease), 188 (25%) required ICU level of care, 133 (18%) required mechanical ventilation, 409 (54%) required supplemental oxygen, and 143 (19%) died within 30 days of COVID-19 diagnosis. Stratified rates of severe outcomes are shown in the Table. The rate of severe COVID-19 was highest in patients with chronic lymphocytic leukemia (53%), and lowest in patients with Hodgkin lymphoma (23%). Patients receiving cytotoxic systemic therapy within 3 months of COVID-19 diagnosis had higher rates of severe COVID-19 (41%) and 30-day mortality (28%) than patients who completed treatment 3-12 months (26% severe COVID-19, 16% 30-day mortality) or more than 12 months (29% severe COVID-19, 13% 30-day mortality) prior to COVID-19 diagnosis. Patients receiving cellular therapy or transplant within a year prior to COVID-19 diagnosis had similar rates of severe COVID-19 (36% v. 38%) and 30-day mortality (23% v. 19%) to patients who had not received such therapies within a year prior to COVID-19 diagnosis. Patients on second-line or later therapy experienced similar rates of poor outcomes (42% severe COVID-19, 20% 30-day mortality) to patients on first-line therapy (39% severe COVID-19, 18% 30-day mortality) and untreated patients (42% severe COVID-19, 20% 30-day mortality). Outcomes for patients receiving therapies under investigation as repurposed COVID-19 treatments were similar to the cohort at large.
Conclusions: This is the largest cohort study to date describing COVID-19 outcomes in patients with HM. Rates of severe COVID-19 outcomes including death were high. Unadjusted rates of severe COVID-19 outcomes were higher in patients with previously described risk factors such as advanced age, poor performance status, and progressive disease, as well as those receiving recent cytotoxic therapy. Outcomes varied widely by malignancy but were similar across treatment contexts. Additional data collection and analyses are ongoing.
Lynch:Bayer: Research Funding; Rhizen Pharmaceuticals: Research Funding; Incyte: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; Juno Therpeutics: Research Funding; Genentech: Research Funding; MorphoSys: Consultancy; Cyteir: Research Funding. Bakouny:BMS: Research Funding; Genentech: Research Funding. Bhutani:Sanofi: Consultancy, Research Funding. Shah:American Cancer Society and the Hope Foundation for Cancer Research: Research Funding; National Cancer Institute: Research Funding. Lyman:Mylan: Consultancy; Beyond Spring: Consultancy; Samsung: Consultancy; Sandoz: Consultancy; Invitae: Consultancy; Spectrum: Consultancy; G1 Therapeutics: Consultancy; Amgen: Research Funding. Kuderer:Janssen: Consultancy; G1 Therapeutics: Consultancy; Beyond Springs: Consultancy; Spectrum Pharmaceuticals: Consultancy; Bayer: Consultancy; Bristol-Myers Squibb: Consultancy; celldex: Consultancy; Total Health: Consultancy; Invitae: Consultancy. Warner:HemOnc.orgLLC: Other: Shareholder/Stockholder/Stock options; National Cancer Institute: Research Funding; IBM Watson Health: Consultancy; Westat: Consultancy. Mesa:Bristol Myers Squibb: Research Funding; Incyte: Research Funding; AbbVie: Research Funding; Samus Therapeutics: Research Funding; Genentech: Research Funding; Promedior: Research Funding; CTI BioPharma: Research Funding; Novartis: Consultancy; Sierra Oncology: Consultancy; LaJolla Pharmaceutical Company: Consultancy. Thompson:AIM Specialty Health, BMS, GlaxoSmithKline, Takeda, Via Oncology: Membership on an entity's Board of Directors or advisory committees; Doximity: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Synapse Precision Medical Council: Other: Travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.